EYE BANKING, CELL THERAPY AND OCULAR REGENERATIVE MEDICINE: OUR PROJECTS
Significant advances have been made in the field of eye banking, cell terapy and ocular regenerative medicine. Fondazione Banca degli Occhi del Veneto (Veneto Eye Bank Foundation) is currently pursuing the following research topics, to evaluate new challenges and new possibilities for biobanking and ocular clinical and therapeutic treatments for patients.

Effect of Different Corneal Preservation Medium and Conditions on Pre-loaded Descemet Membrane Endothelial Keratoplasty (DMEK) Grafts
The project “Effect of Different Corneal Preservation Medium and Conditions on Pre-loaded Descemet Membrane Endothelial Keratoplasty (DMEK) Grafts”, supported by the EBAA “Richard Lindstrom Research Grant” in 2017, aims to determine the viability and biomechanical behaviour of pre-loaded DMEK grafts when preserved in organ culture medium (dextran based) at 31oC and Optisol-¬‐GS at 4oC. The project has been proposed by the principal investigator Mohit Parekh, PhD., Veneto Eye Bank Foundation, and other investigators and collaborators: Alessandro Ruzza, BSc., and Stefano Ferrari, PhD., Veneto Eye Bank Foundation, Venice, Italy , Vito Romano, MD., Moorfields Eye Hospital,London, UK, and Stephen Kaye, MD., University of Liverpool and Royal Liverpool University Hospital, Liverpool, UK. DMEK has shown a higher rehabilitation rate and better visual outcomes, but it is not taken up widely due to the challenges required in preparation and implantation techniques. Tri-folded (endothelium-¬‐in) DMEK grafts preloaded in a single use device and stored up to 4 days are expected to show limited endothelial damage when preserved with medium supplemented with dextran. Working together with eye banks to provide such preloaded DMEK grafts will reduce the cost, preparation issues and tissue wastage and reduce surgical time which will facilitate the uptake of DMEK. The effects of different media and conditions will verify the use of specific medium and conditions for pre-loading DMEK grafts in countries using organ culture system or hypothermic method.

European Cornea and Cell Transplantation Registry (ECCTR)
The European Cornea and Cell Transplantation Registry (ECCTR) is a European Consortium that aims to build an EU web-based registry in the field of cornea and to asses and verify the safety quality and efficacy of (new) human tissue transplantation in ophthalmic surgery.The aim of the project isto build a common assessment methodology and establish an EU web-based registry and network for academics, health professionals and authorities to assess and verify the safety quality and efficacy of (new) human tissue transplantations and in ophthalmic surgery.
The project is a three-year programme, with development of an EU web registry in the first year, followed by recruitment of clinics and eye banks and collection of data in the second year. Evaluation of the collected data and development of an evidence-based European protocol will take place in year three, and results will also disseminated at a final conference. More details can be found at

Euro GTP II Good Tissue and Cell Practices (EuroGTPII)
Good Practices for demonstrating safety and quality through recipient follow-upis a 3 year Project co-funded by the 3rd Health Programme of the European Union under the Grant Agreement number: 709567.This project aims to set up the good practices applied to tissues and cells (T&C) preparation processes and patient follow-up procedures.EuroGTP II intends to provide practical tools which will assist Tissue Establishments and Organizations Responsible for Human Application, in the implementation of technical requirements defined for the assessment and verification of the quality, safety and efficacy of therapies with human T&C. Moreover, these tools will be developed in accordance with the regulatory principles, legislation and good practices, and will be made available to National Competent Authorities (NCAs), hence facilitating also the evaluation and the authorization procedures.
The Barcelona Tissue Bank (Banc de Teixits de Barcelona – Banc de Sang I Teixits(BST)) is the Coordinator of this Project, that involves a total 14 Associative partners from 11 Member States, and 12 Collaborative partners (including Fondazione Banca degliOcchi del Veneto, Fondazione Banca deiTessuti di Treviso and the European Eye Bank Association). More details can be found at

COST Action BM1302Joining forces in Corneal Regeneration Research
Corneal blindness is a worldwide problem and can be treated by transplantation of donor human tissue, or by implantation of artificial, non-biological corneas. However, there is a great lack of donors worldwide and current implants are either expensive or do not integrate well. The solution may be found in the development of biodegradable artificial corneas, consisting of bio-matrices seeded with host limbal stem cells. However, current emerging research is in its early stages and fragmented among research institutes throughout Europe.ThisCOST Actioncombines scientific, clinical and industrial knowledge in the field of corneal regeneration.
This will link researchers in the fields of biomaterial research, stem cell manipulation, tissue engineering, ophthalmology and immunology. This planned collaboration will, not only result in a unique European knowledge base to boost the development of biodegradable artificial corneas from basic research to clinical application, but will also provide the foundation to foster the development of a new generation of cornea specialists that are interested in regenerative medicine, especially in ophthalmology. In regard of the aging population of Europe, such a focus is of great importance to help our increasing population of elderly individuals with corneal disease. More details can be found at
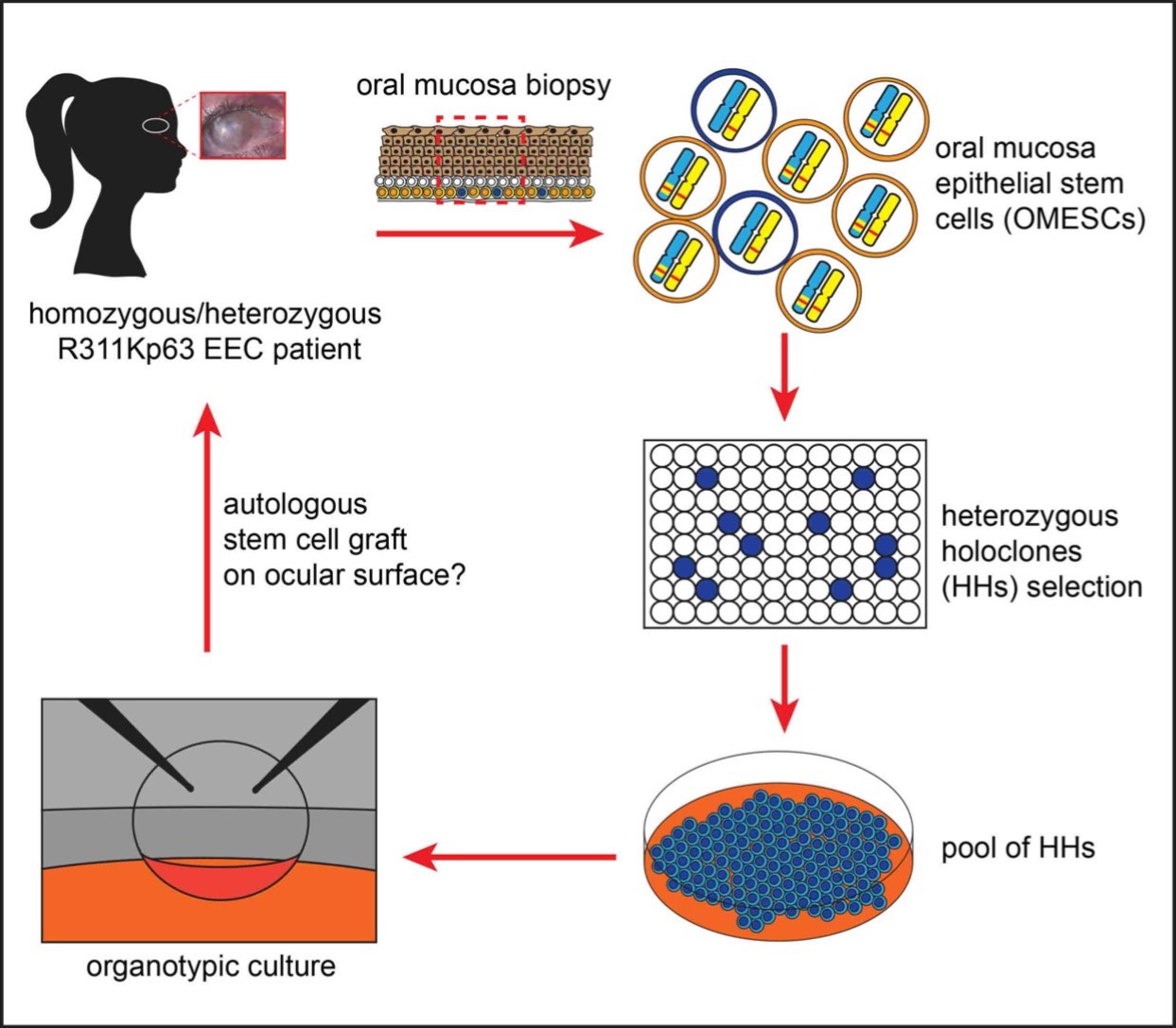
Towards a cell/gene-based therapeutic approach for a rare disorder of the cornea
The Ectrodactyly-Ectodermal dysplasia-Clefting (EEC) syndrome is a rare autosomal dominant inherited disease characterised by ectrodactyly (split-hand-foot malformation), ectodermal dysplasia and cleft lip and palate. It affects the skin, nails, hair, teeth, sweat glands and the ocular ectodermal derivatives. However, the major cause of visual morbidity is limbal stem cell failure, resulting in recurrent corneal ulceration, neovascularisation and spontaneous corneal perforation. The progressive keratopathy associated with limbal stem cell failure results in a dense vascularised corneal pannus, leading to progressive corneal clouding and eventually visual impairment. EEC syndrome is caused by heterozygous missense point mutations in the p63 gene, a “molecular activator” essential for the regeneration of adult epithelia.The aim of this project is to make all the necessary steps towards a clinical study on patients affected by EEC syndrome. It is a collaborative project between Fondazione Banca degliOcchi del Veneto and the Department of Molecular Medicine of the University of Padova. The project is currently funded through a grant from the French Muscular Dystrophy Association (http://www.afm-telethon.com/) and the “5 per Mille” funds of the Ministerodella Salute and Ministerodell’Istruzione, dell’Università e della Ricerca.

